Welcome to the fascinating world of chemistry, where molecular structures hold the key to understanding how substances interact and transform. Today, we’re diving into hcooch ch2 h2o a compound that may not be on everyone’s radar but is packed with potential. This intriguing molecule plays a vital role across various fields, from industrial applications to research advancements. Curious about its chemical structure? Eager to explore its reactions and real-world uses? Stick around as we unravel the mysteries surrounding hcooch ch2 h2o!
Chemical Structure and Properties
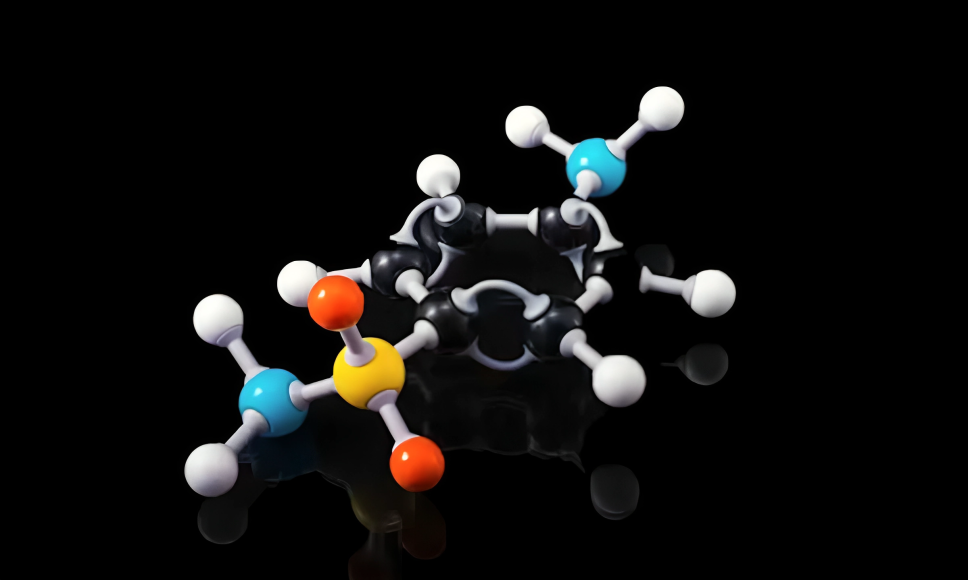
hcooch ch2 h2o commonly known as formic acid methyl ester hydrates the chemical structure is intriguing. It features a carbonyl group bonded to a methoxy group and an alcohol molecule. This arrangement gives it unique properties.
The presence of the carbonyl (C=O) enhances its reactivity, making it suitable for various reactions. The hydroxyl (-OH) group contributes to hydrogen bonding, affecting its solubility in water and organic solvents.
In terms of physical properties, hcooch ch2 h2o exhibits moderate volatility and distinctive odor characteristics. Its molecular weight impacts density and viscosity levels, which are crucial in industrial applications.
These aspects make this compound versatile across different fields while maintaining stability under standard conditions. Understanding these elements lays the groundwork for exploring its reactions and potential uses further down the line.
Reactions of HCOOCH CH₂ H₂O
hcooch ch2 h2o, commonly referred to as formic acid methyl ester, participates in several intriguing chemical reactions. One of the notable reactions involves hydrolysis. In this process, water interacts with the compound, leading to the formation of methanol and formic acid. This dual product generation is vital for various applications.
Another significant reaction is transesterification. When mixed with different alcohols under specific conditions, it can yield esters that are crucial in biodiesel production. These derivatives showcase valuable properties for fuel efficiency.
Oxidation is yet another pathway where hcooch ch2 h2o can be transformed into more complex molecules. This characteristic makes it a useful building block in organic synthesis.
These diverse reactions illustrate its versatility and importance across multiple fields such as chemistry and industrial processes. The potential for innovation remains vast as research continues to explore new pathways and applications.
Applications in Industry and Research
HCOOCH CH₂ H₂O plays a significant role across various industries. Its unique chemical structure allows it to serve as an effective solvent in chemical synthesis and extraction processes.
In pharmaceuticals, this compound aids in formulating drugs, enhancing solubility and bioavailability. Researchers utilize it to study reaction mechanisms due to its reactivity.
The agriculture sector also benefits from hcooch ch2 h2o. It functions as a biodegradable surfactant in pesticides, promoting better adherence to plant surfaces.
Environmental research harnesses the compound for studying pollutant degradation pathways. This highlights its potential in developing eco-friendly strategies.
Moreover, its applications extend to food science where it’s explored for preserving freshness and flavor stability in products. The versatility of hcooch ch2 h2o continues to spark interest among scientists and industry professionals alike.
Safety and Handling of HCOOCH CH₂ H₂O
When working with hcooch ch2 h2o safety is paramount. Always wear appropriate personal protective equipment (PPE), including gloves and goggles. This minimizes exposure to any harmful effects.
Ensure that you’re operating in a well-ventilated space. Fumes can accumulate easily if precautions aren’t taken seriously. A fume hood provides added protection during experiments.
Store this compound in a secure location away from incompatible substances. Follow regulatory guidelines for chemical storage to prevent accidents.
Be aware of emergency procedures should an accidental spill occur. Have absorbent materials on hand for quick cleanup, and familiarize yourself with the Material Safety Data Sheet (MSDS) specific to hcooch ch2 h2o.
Regular training on safe handling practices is essential for all personnel involved in research or industrial applications involving this chemical compound.
Future Potential and Advancements
The future of hcooch ch2 h2o is brimming with possibilities. Researchers are exploring its potential in sustainable chemistry, particularly as a green solvent. This could lead to reduced environmental impact and enhanced efficiency in various reactions.
Innovations in material science are also on the horizon. Scientists aim to utilize this compound for developing new polymers that boast improved properties while remaining eco-friendly.
Moreover, advancements in biochemistry may unlock novel applications within pharmaceuticals. The unique structure of hcooch ch2 h2o can facilitate drug delivery systems that enhance bioavailability.
As technology progresses, so too will our understanding of this compound’s capabilities. Collaborative efforts across multiple disciplines will likely drive exciting breakthroughs, paving the way for practical applications we haven’t yet imagined.
Conclusion
HCOOCH CH₂ H₂O plays a significant role in various fields, from industrial applications to academic research. Understanding its chemical structure and properties provides insights into how it interacts with other substances. The diverse reactions of hcooch ch2 h2o demonstrate its versatility, making it valuable in many processes.
The safety and handling guidelines are crucial for anyone working with this compound. Proper knowledge ensures that risks are minimized while maximizing its potential benefits. As we explore advancements related to hcooch ch2 h2o the future looks promising.
With ongoing research and development, new applications may emerge that can further enhance our understanding of chemistry and benefit numerous industries. Embracing innovations will likely lead to exciting discoveries surrounding this intriguing compound.





